odd electron species|Lewis Theory XIII: Odd Electron Species : Tuguegarao Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule, because the rule requires eight electrons (or two for hydrogen) around each .
There are many ways to remove DRM from WMV files. This tutorial will show you several ways on how to remove DRM protection from WMV files for free. Check it out: 1. Aimersoft DRM Removal Tool Aimersoft Media .
odd electron species,Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be Nitrogen (II) Oxide also called nitric oxide (\(\ce{NO}\). Nitrogen has 5 valence .Odd-electron molecules have an odd number of valence electrons, and therefore have an unpaired electron. Electron-deficient molecules have a central atom that has fewer .Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be .
ChemPhysChem. Article. Free Access. Odd-Electron Bonds. Prof. Dr. Timothy Clark. First published: 24 May 2017. .
ChemTeam: VSEPR - Odd Electron Molecules. VSEPR Structures of Odd Electron Molecules. Back to VSEPR Menu. All the usual rules of building a VSEPR structure will .
Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule, because the rule requires eight electrons (or two for hydrogen) around each .
Odd-electron Molecules. Molecules that contain an odd number of electrons are called radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when .

Lewis Theory XIII: Odd Electron Species. Ben's Chem Videos. 70.8K subscribers. Subscribed. 298. Share. 33K views 12 years ago Lewis Theory. This video .
There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired electrons. . Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be Nitrogen (II) Oxide (NO , refer to figure one). Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used. Piyush Maheshwari and more top educators are teaching live on Unacademy Plus.Use Code “PMSSIR” to get 10% discount on your Unacademy Plus Subscription.Subscr.Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be Nitrogen (II) Oxide (NO , refer to figure one). Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used.complexes. 1. Introduction. Chemists are familiar with the concepts of even (two, four, six, etc.) electron bonds, but far less so with their odd (one, three) electron equivalents. This is mostly because odd-electron bonded species are open shell, and therefore usually kinetically less stable under normal conditions than closed-shell mole-cules.
odd electron speciesThe total number of valence electrons is 5 + 2 (6) = 17. There is the persistent radical character on nitrogen because it has an unpaired electron. C l O 2. A Lewis structure shows C l O 2 has a total of 2 × 6 + 7 = 19 valence electrons, or 9 electron pairs and a lone electron. Cl is the central atom. The molecule C l O 2 has an odd number of . Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be Nitrogen (II) Oxide also called nitric oxide (\(\ce{NO}\). Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used. 1 Introduction. Chemists are familiar with the concepts of even (two, four, six, etc.) electron bonds, but far less so with their odd (one, three) electron equivalents. This is mostly because odd-electron bonded species are open shell, and therefore usually kinetically less stable under normal conditions than closed-shell molecules. Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be nitrogen monoxide also called nitric oxide (\(\ce{NO}\). (1 O atom) x (8) + (1 N atom) x (8) = 16 valence electrons neededLewis Theory XIII: Odd Electron Species Exception 1: Species with Odd Numbers of Electrons. The first exception to the Octet Rule is when there are an odd number of valence electrons. An example of this would be nitrogen monoxide also called nitric oxide (\(\ce{NO}\). (1 O atom) x (8) + (1 N atom) x (8) = 16 valence electrons needed
Welcome to ATP STAR Chemistry channel. This channel is in association with “𝐀𝐓𝐏 𝐒𝐓𝐀𝗥 Kota. Which is India’s Best IIT JEE & NEET Offline/Online Prepara.Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free radical, so we know that not every atom can have eight electrons in its valence shell. Draw a skeleton structure of the molecule.With 5 + 6 = 11 valence electrons, there is no way to draw a Lewis structure that gives each atom an octet of electrons. Molecules such as NO, NO 2, and ClO 2 require a more sophisticated treatment of bonding, .With 5 + 6 = 11 valence electrons, there is no way to draw a Lewis structure that gives each atom an octet of electrons. Molecules such as NO, NO 2, and ClO 2 require a more sophisticated treatment of bonding, which will be developed in Chapter 5. Even without such a treatment, we notice that such odd electron species are extremely reactive. The concept of the odd-electron σ-bond was first proposed by Pauling 8, and species with these intriguing bonds have been recognized as important intermediates in chemistry and biochemistry 9,10 .odd electron species Lewis Theory XIII: Odd Electron Species KO2,N O2,SO2,BaO2,ClO2,O2,N O,N 2O,O3,CO. Q. Assertion: N O2 and CO2 both odd electron molecules and hence dimerizes. Reason :- On dimerisation, N O2 is converted to stable N 2O4 molecule with even number of electrons. View More.
Odd Electron Species: An odd electron species contain an odd number of electrons in its valance shell. Such species violate the octet rule. An odd electron species may also be called a free radical because of having an extra unpaired electron. A free radical is highly reactive and generally exists as a dimer. Answer and Explanation: 1

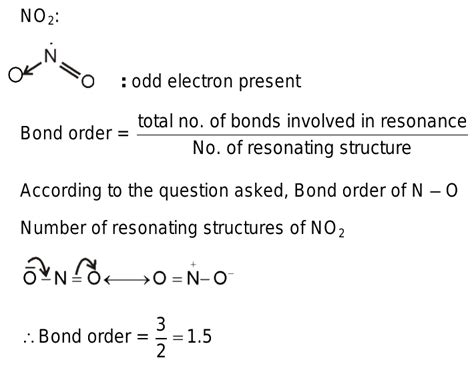
NO2 is an odd electron species. What is the bond order of N – O in NO2 ? Open in App. Solution. Suggest Corrections. 2. Similar questions. Q. The increasing order of O − N − O bond angle in the species N O 2, N O + 2 adn N O . Or we can say that the molecule has unpaired electrons. If any of the atoms has an odd number of valence electrons then the molecule will have odd electrons bond. Complete step by step answer: An odd electron bond means there is an odd number of the electron in the overall molecule. Or we can say that the molecule has unpaired .
All electrons can not be paired up and there is an odd electron responsible for magnetism. N O 2 molecule is therefore paramagnetic. Number of valance electrons in O 2 = 6 + 6 = 12. . Overall 4 species are paramagnetic including N O, N O 2, O 2 and C O + Was this answer helpful? 6. Similar Questions. Q1.
odd electron species|Lewis Theory XIII: Odd Electron Species
PH0 · Violations of the Octet Rule
PH1 · Odd‐Electron Bonds
PH2 · Odd
PH3 · Lewis Theory XIII: Odd Electron Species
PH4 · Exceptions to the Octet Rule
PH5 · ChemTeam: VSEPR
PH6 · 8.7: Exceptions to the Octet Rule
PH7 · 6.5: Exceptions to the Octet Rule